COVID-19
COVID-19 (coronavirus disease) is an infectious respiratory disease caused by the SARS-CoV-2 virus. It can be very contagious and spread quickly to anyone infected with the virus. While most people infected with COVID-19 will experience mild to moderate symptoms and recover without needed medical treatment, some people will become seriously ill and require medical attention. Unfortunately, some people who become seriously ill will succumb to the disease. Thankfully, there are several COVID-19 vaccines authorized and available for use in Canada. The best way to help slow transmission of and protect you from serious COVID-19 illness is by getting immunized.
Check out our resources on COVID-19 immunization and share with your network!
COVID-19 Vaccine Factsheet
COVID-19 Vaccine Videos
COVID-19 Vaccine Posters: One Visit. Two Shots.
One Visit. Two Shots. Protected | Co-administration of influenza and COVID-19 vaccines
(poster 1 – PDF: 3.2 MB)
One Visit. Two Shots. Protected | Co-administration of influenza and COVID-19 vaccines
(poster 2 – PDF: 2.4 MB)
One Visit. Two Shots. Protected | Co-administration of influenza and COVID-19 vaccines
(poster 4 – PDF: 2.1 MB)
One Visit. Two Shots. Protected | Co-administration of influenza and COVID-19 vaccines
(poster 5 – PDF: 4.0 MB)
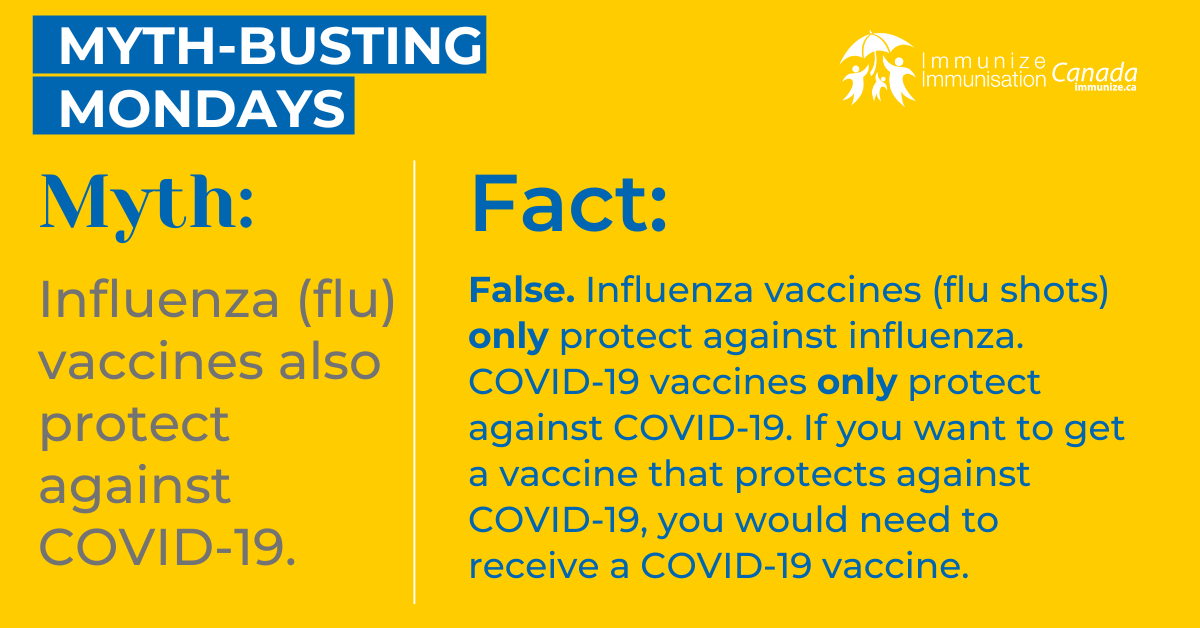
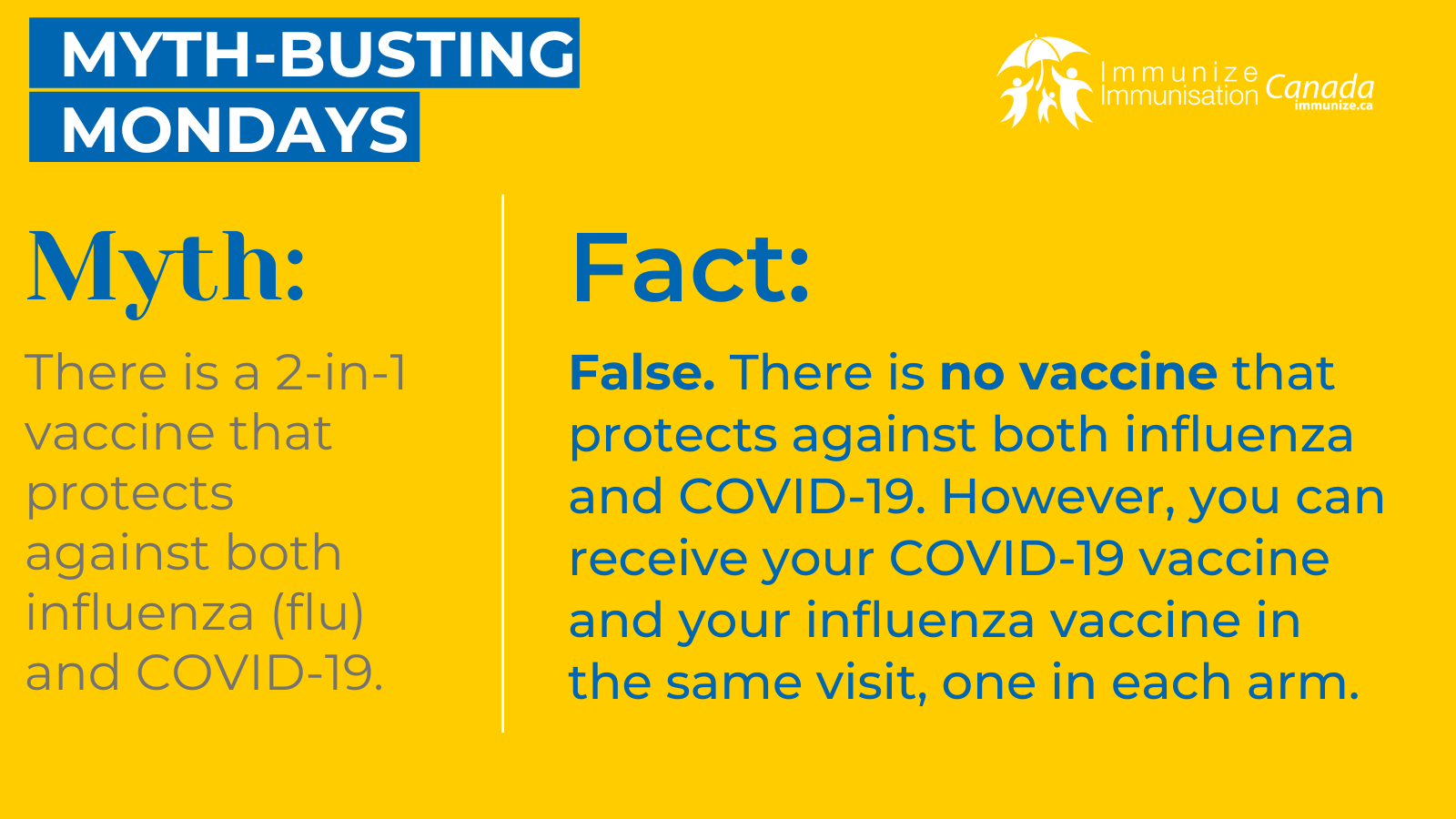
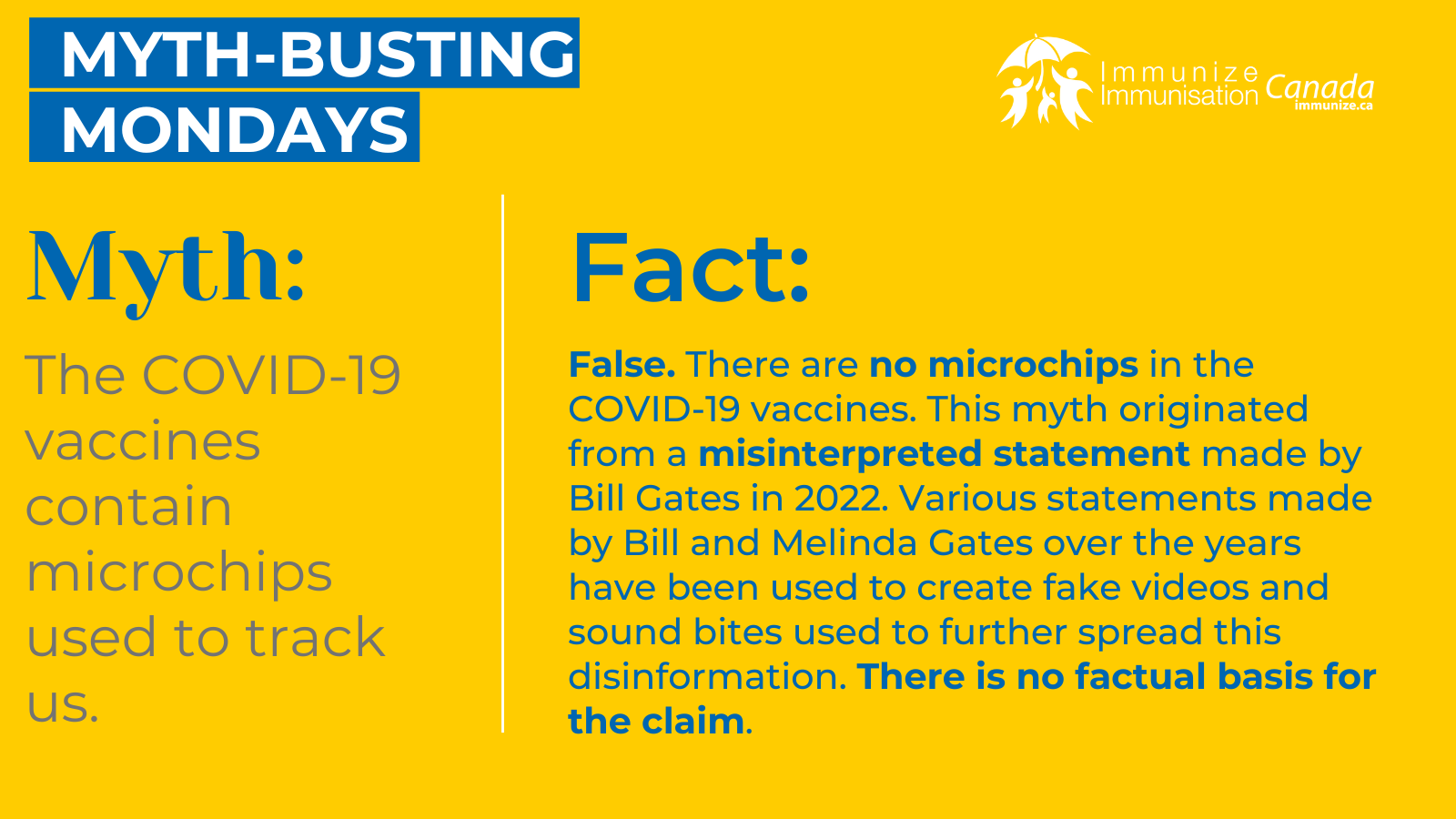
COVID-19 Vaccine Social Media Images: Myth-busting Mondays
Myth-busting Monday
(image 13 for Twitter/X – .png: 289 KB)

COVID-19 Vaccine Social Media Images: One Visit. Two Shots.
One Visit. Two Shots. Protected | Co-administration of influenza and COVID-19 vaccines
(image for Twitter 1 – .png: 1.2 MB)
One Visit. Two Shots. Protected | Co-administration of influenza and COVID-19 vaccines
(image for Facebook 1 – .png: 753 KB)
One Visit. Two Shots. Protected | Co-administration of influenza and COVID-19 vaccines
(image for Twitter 2 – .png: 1.2 MB)
One Visit. Two Shots. Protected | Co-administration of influenza and COVID-19 vaccines
(image for Facebook 2 – .png: 603 KB)
One Visit. Two Shots. Protected | Co-administration of influenza and COVID-19 vaccines
(image for Twitter 3 – .png: 1.5 MB)
One Visit. Two Shots. Protected | Co-administration of influenza and COVID-19 vaccines
(image for Facebook 3 – .png: 875 KB)
One Visit. Two Shots. Protected | Co-administration of influenza and COVID-19 vaccines
(image for Twitter 4 – .png: 1.1 MB)
One Visit. Two Shots. Protected | Co-administration of influenza and COVID-19 vaccines
(image for Facebook 4 – .png: 613 KB)
One Visit. Two Shots. Protected | Co-administration of influenza and COVID-19 vaccines
(image for Instagram 4 – .png: 1.2 MB)

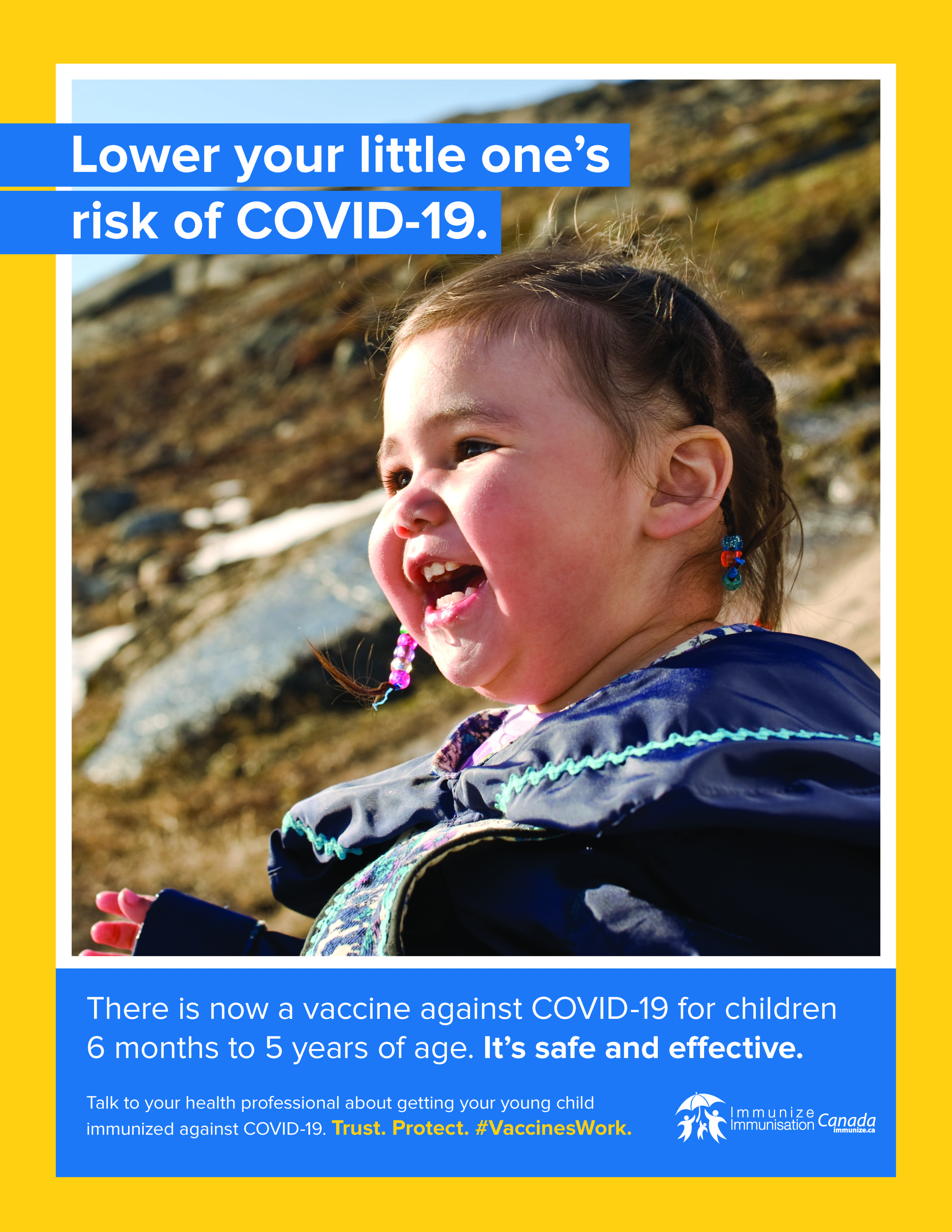
COVID-19 vaccine awareness posters and social media images
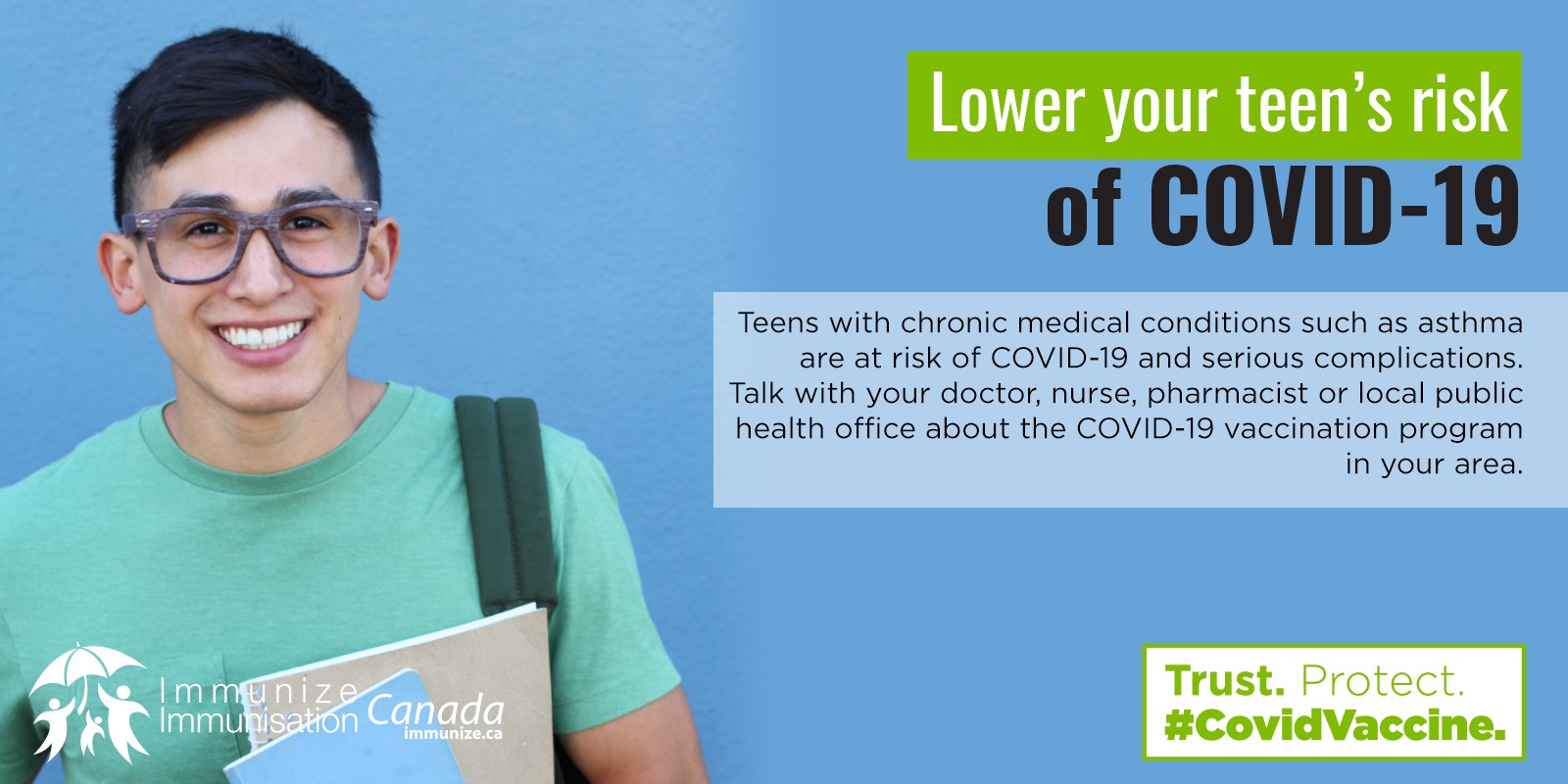
Lower your risk of COVID-19: people with chronic medical conditions
(social media image – .jpg: 188 KB)
NACI Guidance
-
Updated chapter – COVID-19 vaccines: Canadian Immunization Guide. Public Health Agency of Canada. 19 September 2025.
-
Updated chapter – COVID-19 vaccines: Canadian Immunization Guide. Public Health Agency of Canada. 05 February 2025.
-
Updated chapter – COVID-19 vaccines: Canadian Immunization Guide. Public Health Agency of Canada. 27 September 2024.
-
Guidance on the use of COVID-19 vaccines during the fall of 2024. National Advisory Committee on Immunization (NACI). 03 May 2024.
-
Summary of NACI statement of May 3, 2024: Guidance on the use of COVID-19 vaccines during the fall of 2024. National Advisory Committee on Immunization (NACI). 03 May 2024.
-
Updated guidance on the use of protein subunit COVID-19 vaccine (Novavax Nuvaxovid). National Advisory Committee on Immunization (NACI). 08 March 2024.
-
Summary of National Advisory Committee on Immunization (NACI) Statement of March 8, 2024: Updated guidance on the use of protein subunit COVID-19 vaccine (Novavax Nuvaxovid). National Advisory Committee on Immunization (NACI). 08 March 2024.
-
Guidance on an additional dose of COVID-19 vaccines in the spring for individuals at high risk of severe illness due to COVID-19. National Advisory Committee on Immunization (NACI). 12 January 2024.
- Summary of NACI Statement of January 12, 2024: Guidance on an additional dose of COVID-19 vaccines in the spring for individuals at high risk of severe illness due to COVID-19. National Advisory Committee on Immunization (NACI). 12 January 2024.
- Updated chapter – COVID-19 vaccines: Canadian Immunization Guide. Public Health Agency of Canada. 05 December 2023.
- Updated guidance on the use of COVID-19 vaccines in individuals who have not previously been vaccinated against COVID-19. National Advisory Committee on Immunization. 27 October 2023.
- Summary of National Advisory Committee on Immunization (NACI) Statement of October 27, 2023: Updated guidance on the use of COVID-19 vaccines in individuals who have not previously been vaccinated against COVID-19. National Advisory Committee on Immunization. 27 October 2023.
- COVID-19 Vaccines: Canadian Immunization Guide. Public Health Agency of Canada. Updated 20 October 2023.
- Addendum to the guidance on the use of COVID-19 vaccines in the fall of 2023. National Advisory Committee on Immunization (NACI). 12 September 2023.
- Summary of National Advisory Committee on Immunization (NACI) Supplemental Statement of September 12, 2023: Addendum to the guidance on the use of COVID-19 vaccines in the fall of 2023. National Advisory Committee on Immunization (NACI). 12 September 2023.
- Guidance on the use of COVID-19 vaccines in the fall of 2023. National Advisory Committee on Immunization (NACI). 11 July 2023.
- Summary of National Advisory Committee on Immunization (NACI) Statement of July 11, 2023: Guidance on the use of COVID-19 vaccines in the fall of 2023. National Advisory Committee on Immunization (NACI). 11 July 2023.
- Interim guidance on the use of bivalent Omicron-containing COVID-19 vaccines for primary series. National Advisory Committee on Immunization (NACI). 09 June 2023.
- Summary of National Advisory Committee on Immunization (NACI) Statement of June 9, 2023: Interim guidance on the use of bivalent Omicron-containing COVID-19 vaccines for primary series. National Advisory Committee on Immunization (NACI). 09 June 2023.
- Interim guidance on the use of bivalent Omicron-containing COVID-19 vaccines for primary series. National Advisory Committee on Immunization (NACI). 09 June 2023.
- COVID-19 vaccine: Canadian Immunization Guide. Public Health Agency of Canada. Updated 22 March 2023.
- Guidance on an additional COVID-19 booster dose in the spring of 2023 for individuals at high risk of severe illness due to COVID-19. National Advisory Committee on Immunization (NACI). 03 March 2023.
- Summary of National Advisory Committee on Immunization (NACI) Statement of March 3, 2023: Guidance on an additional COVID-19 booster dose in the spring of 2023 for individuals at high risk of severe illness due to COVID-19. National Advisory Committee on Immunization (NACI). 03 March 2023.
- Guidance on COVID-19 vaccine booster doses: Initial considerations for 2023. National Advisory Committee on Immunization (NACI). 20 January 2023.
- Summary of National Advisory Committee on Immunization (NACI) Statement of January 20, 2023: Guidance on COVID-19 vaccine booster doses: Initial considerations for 2023. National Advisory Committee on Immunization (NACI). 20 January 2023.
- Updated recommendations on the use of COVID-19 vaccine booster doses in children 5 to 11 years of age and concurrent vaccine administration. National Advisory Committee on Immunization (NACI). 09 December 2022.
- Summary of National Advisory Committee on Immunization (NACI) Statement of December 9, 2022: Updated recommendations on the use of COVID-19 vaccine booster doses in children 5 to 11 years of age and concurrent vaccine administration. National Advisory Committee on Immunization (NACI). 09 December 2022.
- Summary of National Advisory Committee on Immunization (NACI) updates of November 3, 2022: Recommendations on the use of Moderna Spikevax BA.4/5 bivalent mRNA (50 mcg) COVID-19 booster vaccine in adults. National Advisory Committee on Immunization (NACI). 03 November 2022.
- Recommendations on the use of Pfizer-BioNTech Comirnaty (3 mcg) COVID-19 vaccine in children 6 months to 4 years of age. National Advisory Committee on Immunization (NACI). 21 October 2022.
- Summary of National Advisory Committee on Immunization (NACI) Statement of October 21, 2022: Recommendations on the use of Pfizer-BioNTech Comirnaty (3 mcg) COVID-19 vaccine in children 6 months to 4 years of age. National Advisory Committee on Immunization (NACI). 21 October 2022.
- Updated guidance on COVID-19 vaccine booster doses in Canada. National Advisory Committee on Immunization (NACI). 07 October 2022.
- Summary of National Advisory Committee on Immunization (NACI) Statement of October 7, 2022: Updated guidance on COVID-19 vaccine booster doses in Canada. National Advisory Committee on Immunization (NACI). 07 October 2022.
- Updated guidance on COVID-19 vaccines for individuals who are pregnant or breastfeeding. National Advisory Committee on Immunization (NACI). 09 September 2022.
- Summary of National Advisory Committee on Immunization (NACI) Statement of September 9, 2022: Updated guidance on COVID-19 vaccines for individuals who are pregnant or breastfeeding. National Advisory Committee on Immunization (NACI). 09 September 2022.
- Recommendations on the use of bivalent Omicron-containing mRNA COVID-19 vaccines. National Advisory Committee on Immunization (NACI). 01 September 2022.
- Summary of National Advisory Committee on Immunization (NACI) Statement of September 1, 2022: Recommendations on the use of bivalent Omicron-containing mRNA COVID-19 vaccines. National Advisory Committee on Immunization (NACI). 01 September 2022.
- Recommendations on the use of of a first booster dose of Pfizer-BioNTech Comirnaty COVID-19 vaccine in children 5 to 11 years of age. National Advisory Committee on Immunization (NACI). 19 August 2022.
- Summary of National Advisory Committee on Immunization (NACI) Statement of August 19, 2022: Recommendations on the use of a first booster dose of Pfizer-BioNTech Comirnaty COVID-19 vaccine in children 5 to 11 years of age. National Advisory Committee on Immunization (NACI). 19 August 2022.
- Recommendations on the use of Moderna Spikevax COVID-19 vaccine in children 6 months to 5 years of age. National Advisory Committee on Immunization (NACI). 14 July 2022.
- Summary of National Advisory Committee on Immunization (NACI) Statement of July 14, 2022: Recommendations on the use of Moderna Spikevax COVID-19 vaccine in children 6 months to 5 years of age. National Advisory Committee on Immunization (NACI). 14 July 2022.
- Statement: Health Canada authorizes use of Moderna COVID-19 vaccine in children 6 months to 5 years of age. Health Canada. 14 July 2022.
- Interim guidance on planning considerations for a fall 2022 COVID-19 vaccine booster program in Canada. National Advisory Committee on Immunization (NACI). 29 June 2022.
- Summary of National Advisory Committee (NACI) Statement of June 29, 2022: Interim guidance on planning considerations for a fall 2022 COVID-19 vaccine booster program in Canada. National Advisory Committee on Immunization (NACI). 29 June 2022.
- Summary of updates in the Canadian Immunization Guide of June 21, 2022: Updated guidance on COVID-19 vaccines in Canada. National Advisory Committee on Immunization (NACI). 21 June 2022.
- Updated guidance on a first booster dose of COVID-19 vaccines in Canada. National Advisory Committee on Immunization (NACI). 12 April 2022.
- Summary of National Advisory Committee on Immunization (NACI) Statement of April 12, 2022: Updated guidance on a first booster dose of COVID-19 vaccines in Canada. National Advisory Committee on Immunization (NACI). 12 April 2022.
- Initial guidance on a second booster dose of COVID-19 vaccines in Canada. National Advisory Committee on Immunization (NACI). 05 April 2022.
- Summary of National Advisory Committee on Immunization (NACI) Statement of April 5, 2022: Initial guidance on a second booster dose of COVID-19 vaccine in Canada. National Advisory Committee on Immunization (NACI). 05 April 2022.
- Recommendations on the use of Moderna Spikevax COVID-19 vaccine in children 6 to 11 years of age. National Advisory Committee on Immunization (NACI). 17 March 2022.
- Summary of National Advisory Committee on Immunization (NACI) Statement of March 17, 2022: Recommendations on the use of Moderna Spikevax COVID-19 vaccine in children 6 to 11 years of age. National Advisory Committee on Immunization (NACI). 17 March 2022.
- Recommendations on the use of Medicago COVID-19 vaccine (Covifenz). National Advisory Committee on Immunization (NACI). 11 March 2022.
- Summary of National Advisory Committee on Immunization (NACI) Statement of March 11, 2022: Recommendations on the use of Medicago COVID-19 vaccine (Covifenz). National Advisory Committee on Immunization (NACI). 11 March 2022.
- COVID-19: Recommendations for those vaccinated with vaccines not authorized by Health Canada for those staying in Canada to live, work or study. Public Health Agency of Canada. 11 March 2022.
- Recommendations on the use of Novavax Nuvaxovid COVID-19 vaccine. National Advisory Committee on Immunization (NACI). 17 February 2022.
- Summary of National Advisory Committee on Immunization (NACI) Statement of February 17, 2022: Recommendations on the use of Novavax Nuvaxovid COVID-19 vaccine. National Advisory Committee on Immunization (NACI). 17 February 2022.
- COVID-19 vaccine: Canadian Immunization Guide. Government of Canada. 09 February 2022.
- Rapid response: Updated guidance on COVID-19 vaccination timing for individuals previously infected with SARS-CoV-2. National Advisory Committee on Immunization (NACI). 04 February 2022.
- Summary of National Advisory Committee on Immunization Rapid Response of February 4, 2022. National Advisory Committee on Immunization (NACI). 04 February 2022.
- Rapid response: Guidance on the use of booster COVID-19 vaccine doses in adolescents 12-17 years of age. National Advisory Committee on Immunization (NACI). 28 January 2022.
- Summary of National Advisory Committee on Immunization Rapid Response of January 28, 2022. National Advisory Committee on Immunization (NACI). 28 January 2022.
- Updated recommendations on the use of COVID-19 vaccines in children 5 to 11 years of age. National Advisory Committee on Immunization (NACI). 25 January 2022.
- Summary of National Advisory Committee on Immunization Statement of January 25, 2022 – Updated recommendations on the use of COVID-19 vaccines in children 5 to 11 years of age. National Advisory Committee on Immunization (NACI). 25 January 2022.
- Summary of NACI advice on vaccination with COVID-19 vaccines following myocarditis (with or without pericarditis). National Advisory Committee on Immunization (NACI). 14 January 2022.
- Guidance on booster COVID-19 vaccine doses in Canada – Update December 3, 2021. National Advisory Committee on Immunization (NACI). 03 December 2021.
- Summary of the National Advisory Committee on Immunization (NACI) Statement of December 3, 2021. National Advisory Committee on Immunization (NACI). 03 December 2021.
- Rapid response: Updated recommendation on the use of authorized COVID-19 vaccines in individuals aged 12 years and older in the context of myocarditis and pericarditis reported following mRNA COVID-19 vaccines. National Advisory Committee on Immunization (NACI). 03 December 2021.
- Summary of the National Advisory Committee on Immunization (NACI) Rapid Response of December 3, 2021. National Advisory Committee on Immunization (NACI). 03 December 2021.
- Recommendation on the use of the Pfizer-BioNTech COVID-19 vaccine (10 mcg) in children 5-11 years of age. National Advisory Committee on Immunization (NACI). 19 November 2021.
- Summary of the National Advisory Committee on Immunization (NACI) Statement of November 19, 2021 – Recommendation on the use of the Pfizer-BioNTech COVID-19 vaccine (10 mcg) in children 5-11 years of age. National Advisory Committee on Immunization (NACI). 19 November 2021.
- Interim guidance on booster COVID-19 vaccine doses in Canada. National Advisory Committee on Immunization (NACI). 29 October 2021.
- Summary of National Advisory Committee on Immunization (NACI) Statement: Interim guidance on booster COVID-19 vaccine doses in Canada. National Advisory Committee on Immunization (NACI). 29 October 2021.
- Recommendations on the use of COVID-19 vaccines. National Advisory Committee on Immunization (NACI). 22 October 2021.
- Summary of National Advisory Committee on Immunization (NACI) statement: Recommendations on the use of COVID-19 vaccines – update: October 22, 2021. National Advisory Committee on Immunization (NACI). 22 October 2021.
- Quick reference guide on use of COVID-19 vaccines: Overview. Public Health Agency of Canada. 13 October 2021.
- National Advisory Committee on Immunization (NACI) rapid response: Booster dose in long-term care residents and seniors living in other congregate settings. National Advisory Committee on Immunization (NACI). 28 September 2021.
- Summary of National Advisory Committee on Immunization (NACI) rapid response: Booster dose of COVID-19 vaccine in long-term care residents and seniors living in other congregate settings. National Advisory Committee on Immunization (NACI). 28 September 2021.
- National Advisory Committee on Immunization (NACI) rapid response: Additional dose of COVID-19 vaccine in immunocompromised individuals following 1- or 2- dose primary series. National Advisory Committee on Immunization (NACI). 10 September 2021.
- Summary of National Advisory Committee on Immunization (NACI) rapid response: Additional dose of COVID-19 vaccine in immunocompromised individuals following a 1- or 2-dose primary series. National Advisory Committee on Immunization (NACI). 10 September 2021.
- Recommendation on the use of mRNA COVID-19 vaccines in adolescents 12 to 17 years of age. National Advisory Committee on Immunization (NACI). 27 August 2021.
- COVID-19 Recommendations for those vaccinated with vaccines not authorized by Health Canada for those staying in Canada to live, work or study. Government of Canada. 17 August 2021.
- NACI Rapid Response: Interchangeability of authorized COVID-19 vaccines. National Advisory Committee on Immunization (NACI). 01 June 2021.
- Summary of NACI Rapid Response of June 1, 2021. National Advisory Committee on Immunization (NACI). 01 June 2021.
- Use of Pfizer-BioNTech COVID-19 vaccine in adolescents 12-18 years of age. National Advisory Committee on Immunization (NACI).
- Summary of National Advisory Committee on Immunization Statement of May 18, 2021. National Advisory Committee on Immunization (NACI). 18 May 2021.
- Extended dose intervals for COVID-19 vaccines to optimize early vaccine rollout and population protection in Canada in the context of limited vaccine supply. National Advisory Committee on Immunization (NACI). 07 April 2021.
- Summary of NACI extended dose intervals statement of April 7, 2021. National Advisory Committee on Immunization (NACI). 07 April 2021.
- COVID-19 in Canada: A one-year update on social and economic impacts. Statistics Canada. 11 March 2021.
- Guidance on the prioritization of initial doses of COVID-19 vaccine(s). National Advisory Committee on Immunization (NACI).
- Interim guidance on continuity of immunization programs during the COVID-19 pandemic. National Advisory Committee on Immunization (NACI).
- Recommendations on the use of COVID-19 vaccines. National Advisory Committee on Immunization (NACI).
- Preliminary guidance on key populations for early COVID-19 immunization. National Advisory Committee on Immunization (NACI).
- Planning guidance for administration of COVID-19 vaccine. National Advisory Committee on Immunization (NACI).
- Planning guidance for immunization clinics for COVID-19 vaccines. National Advisory Committee on Immunization (NACI).
- Vaccines and treatments for COVID-19: Vaccine rollout. Government of Canada.
- Coronavirus disease: Guidance documents. Government of Canada.
Additional Resources
- COVID-19 vaccine for children and adolescents. Canadian Paediatric Society Position Statement. 25 September 2023.
- Let’s Talk COVID Vaccines. Institute for Vaccine Safety. Johns Hopkins Bloomberg School of Public Health | Immunize Canada.
- COVID-19 in young children. COVID-19 Vaccine for Kids Under 5 video series. Children’s Healthcare Canada and Solutions for Kids in Pain (SKIP). July 2022.
- Is the COVID-19 vaccine for children under 5 considered experimental? COVID-19 Vaccine for Kids Under 5 video series. Children’s Healthcare Canada and Solutions for Kids in Pain (SKIP). July 2022.
- How soon after a first dose of the COVID-19 vaccine should a child under 5 receive a second dose, and does that change if they have recently been infected with COVID-19? COVID-19 Vaccine for Kids Under 5 video series. Children’s Healthcare Canada and Solutions for Kids in Pain (SKIP). July 2022.
- Is it safer and more effective, in terms of protection from infection and impacts for children younger than 5, to gain immunity from a COVID-19 vaccine, or through a COVID-19 infection? COVID-19 Vaccine for Kids Under 5 video series. Children’s Healthcare Canada and Solutions for Kids in Pain (SKIP). July 2022.
- What do we know about the risks to children younger than 5 of catching COVID-19 and experiencing severe short- or long-term outcomes? COVID-19 Vaccine for Kids Under 5 video series. Children’s Healthcare Canada and Solutions for Kids in Pain (SKIP). July 2022.
- What do we know about the effectiveness of the COVID-19 vaccine for children younger than 5, in terms of preventing infection and severe effects of COVID-19? COVID-19 Vaccine for Kids Under 5 video series. Children’s Healthcare Canada and Solutions for Kids in Pain (SKIP). July 2022.
- All parents want to do what is best for their children, including of course those younger than 5. In terms of COVID-19, what is your best advice, informed by the current state of the science, for these parents? COVID-19 Vaccine for Kids Under 5 video series. Children’s Healthcare Canada and Solutions for Kids in Pain (SKIP). July 2022.
- Is COVID-19 mild for kids? Video by Science Up First. 13 July 2022.
- Kids & Vaccines Town Hall. Science Up First. Recorded 27 January 2022.
- What are the benefits of vaccinating my child against COVID-19? Video by Health Canada. 28 January 2022.
- How are children’s COVID-19 vaccines monitored for safety and side effects? Video by Health Canada. 28 January 2022.
- My child turns 12 soon. Should they wait to receive the dose for ages 12 and up? Video by Health Canada. 28 January 2022.
- Kids and Omicron (recording of virtual town hall). Children’s Hospital of Eastern Ontario (CHEO). 06 January 2022.
- How COVID-19 mRNA vaccines work. Video by Vaccine Makers Project.
- How COVID-19 viral vector vaccines work. Video by Vaccine Makers Project.
- COVID-19: Vaccine safety post-market surveillance. Healthy Canadians.
- Vaccine Ambassador Stories: Hannah (English). Ottawa Public Health.
- Vaccine Ambassador Stories – Sheekoyinka Safiirada Tallaalka COVID-19 Hannah (Somali). Ottawa Public Health.
- Vaccine Ambassador Stories: Félicité (English). Ottawa Public Health.
- Vaccine Ambassador Stories: Félicité (French). Ottawa Public Health.
- #MyWhy on Getting the COVID-19 Vaccine: Dr. Theresa Tam, Chief Public Health Officer of Canada.
- #MyWhy on Getting the COVID-19 Vaccine: Dr. Howard Njoo, Deputy Chief Public Health Officer of Canada.
- #MyWhy on Getting the COVID-19 Vaccine: Dr. Guillaume Poliquin, Acting Vice President, National Microbiology Laboratory.
- #MyWhy on Getting the COVID-19 Vaccine: Dr. Supriya Sharma, Chief Medical Advisor, Health Canada.
- #MyWhy on Getting the COVID-19 Vaccine: Angela Spence-Bédard, Nurse Practitioner.
- #MyWhy on Getting the COVID-19 Vaccine: Dr. Noni E. MacDonald, Professor of Pediatrics, Infectious Diseases.
- #MyWhy on Getting the COVID-19 Vaccine: Dr. Kal Belay, Acute Care and Minimally Invasive General Surgeon.
- Ask the Experts video series: COVID-19 vaccines questions. Government of Canada.
- COVID-19 awareness resources and videos in Indigenous languages. Public Health Agency of Canada, Indigenous Services Canada and various Indigenous organizations.
- Pediatricians discuss vaccines, pregnancy, development and autism (video). 23 December 2021. The Children’s Hospital of Philadelphia (US).
- Vaccine Conversations: Why do unvaccinated people pose a risk to vaccinated people? (video) 10 December 2021. The Children’s Hospital of Philadelphia (US).
- What we know about vaccines, the immune system and autoimmunity (video). 24 November 2021.
- Vaccine Conversations: Vaccines and breastfeeding (video). 24 November 2021. The Children’s Hospital of Philadelphia (US).
- Wondering why you should get the COVID-19 vaccine? Families Against COVID-19 (US).
- Why I got the COVID-19 vaccine: Isabel. Vaccine Education Center, Children’s Hospital of Philadelphia (US).
- Why I got the COVID-19 vaccine: Jason. Vaccine Education Center, Children’s Hospital of Philadelphia (US).
- Why I got the COVID-19 vaccine and what I felt afterward: Ritter. Vaccine Education Center, Children’s Hospital of Philadelphia (US).
- Why I got the COVID-19 vaccine and what I felt afterward: Victoria. Vaccine Education Center, Children’s Hospital of Philadelphia (US).
- Why I got the COVID-19 vaccine and what I felt afterward: Aleshia. Vaccine Education Center, Children’s Hospital of Philadelphia (US).
- Why I got the COVID-19 vaccine: Gilbert. Vaccine Education Center, Children’s Hospital of Philadelphia (US).
- Why I got the COVID-19 vaccine: Kristen. Vaccine Education Center, Children’s Hospital of Philadelphia (US).
- Why I got the COVID-19 vaccine: Paul. Vaccine Education Center, Children’s Hospital of Philadelphia (US).
- Why I got the COVID-19 vaccine: Safa. Vaccine Education Center. Children’s Hospital of Philadephia (US).
- Why I got the COVID-19 vaccine and what I felt afterward: Tyra. Vaccine Education Center, Children’s Hospital of Philadelphia (US).
- COVID-19 Social Media Graphics: #StopTheSpread. National Foundation for Infectious Diseases (US).
- COVID-19 Social Media Graphics: COVID-19 Myths and Facts. National Foundation for Infectious Diseases (US).
- COVID-19 Social Media Graphics: General Myths and Facts. National Foundation for Infectious Diseases (US).
- COVID-19 Social Media Graphics: #StopTheSpread. National Foundation for Infectious Diseases (US).
- COVID-19 Social Media Graphics: COVID-19 Myths and Facts. National Foundation for Infectious Diseases (US).
- COVID-19 Social Media Graphics: General Myths and Facts. National Foundation for Infectious Diseases (US).